By Leila S. Lemos, Ph.D. candidate in Wildlife Sciences, Fisheries and Wildlife Department
What did you do when playing hide-and-seek? You would try your best not to move or make any noise that would cause the seeker to hear you and find you, right? So, I always associated the prey-predator relationship to a hide-and-seek game, where prey hide, and predators seek. Thus, if you are the prey in this food chain game you should try to hide and not make any noise.
I read an article last week that made me think of this relationship again. The article, “Right whale moms ‘whisper’ to their babies so sharks won’t hear”, announced the study findings from Susan E. Parks and collaborators (2019), which really called my attention.
To give some context, North Atlantic Right Whales (NARWs; Eubalaena glacialis; Fig. 1) occur primarily in northern Atlantic coastal waters or close to the continental shelf (Fig. 2), yet their presence in deep waters are also known (NOAA 2019).

Source: Dana Cusano, Syracuse University (NMFS Permit #775-1875); retrieved from Kooser 2019.

Source: NOAA 2019.
The species is critically endangered and estimated at less than 500 individuals (IUCN 2007, Pace et al. 2017). Unlike several other whale populations, NARWs have not rebounded from intense whaling, and its population has begun to decrease since 2010 (Thomas et al. 2016, Pace et al. 2017). NARWs’ biggest threats are associated with anthropogenic activities, including entanglement in fishing lines and collisions with vessels (Fig. 3).
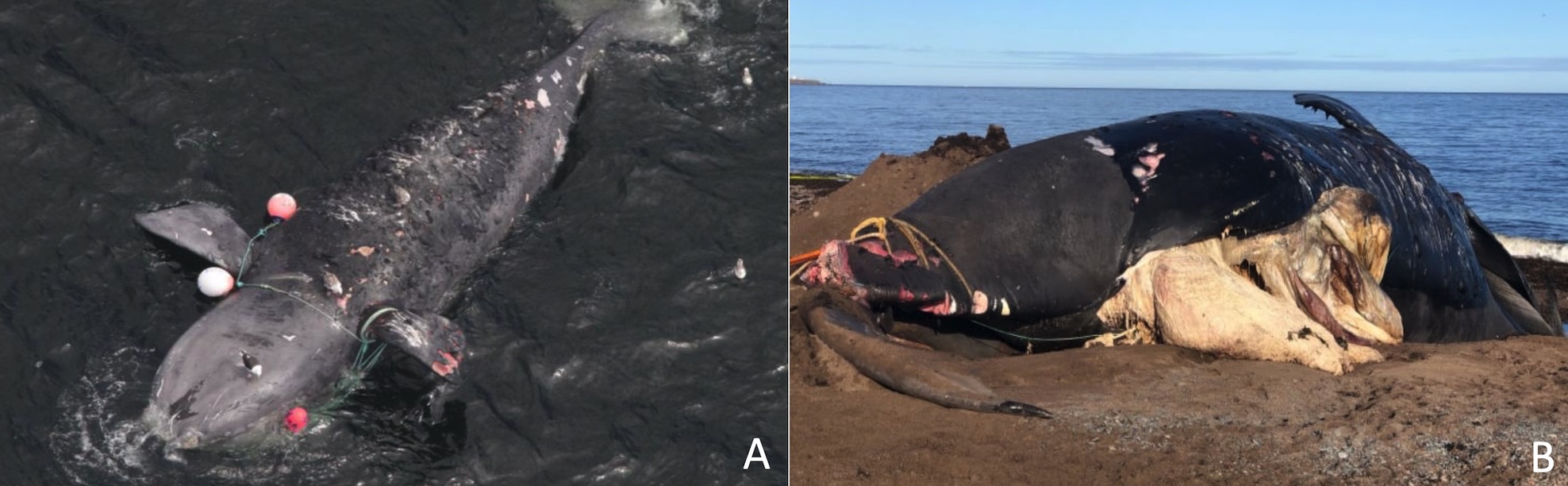
Source (A): Peter Duley (NOAA), retrieved from Guy 2017; (B) Williams 2019.
Other than anthropogenic impacts, NARWs also face natural threats like predation. There are reports on newborn and young right whale calf’s predation by killer whales and large sharks (Taylor et al. 2013, Parks et al. 2019; Fig. 4).

Source: Taylor et al. 2013.
Whales communicate by acoustic signals that can efficiently propagate underwater and be detected by listening predators (Parks et al. 2019). It is possible that mother-calf pairs may use cryptic behaviors to avoid the attention of predators by shifting their communication patterns, leading to a hypothesis that they produce low-amplitude calls and lower call rates (Tyack 2000; Fig. 5). These two behavioral modifications have been previously observed in mother-calf pairs of humpback whales (Megaptera novaeangliae; Videsen et al. 2017) and southern right whales (Eubalaena australis; Nielsen et al. 2019).

Source: Parks et al. 2019.
In order to determine if NARWs exhibited the same behavior, Parks and collaborators (2019) tagged lactating and non-lactating females, and a pregnant female that later was tagged again with her calf, to collect acoustic, movement and orientation data. Their results indicate that lactating females use a significantly higher low-amplitude call rate (mean ± standard deviation: 7.13 ± 2.0 calls) when compared to high-amplitude calls (0.88 ± 0.70 calls). In contrast, non-lactating females exhibited higher rates of high-amplitude calls (3.21 ± 2.29 calls) and lower rates of calls of low-amplitude (0.80 ± 1.15 calls).
Even though their sample size was small (n = 16), the authors had more lactating females sampled than the other demographic groups (n = 11), and their results provide evidence that right whale mother-calf pairs exhibit a shift in their repertoire: Mother-calf pairs reduce high-amplitude calls as compared with other demographic groups in the same habitat (Fig. 6).
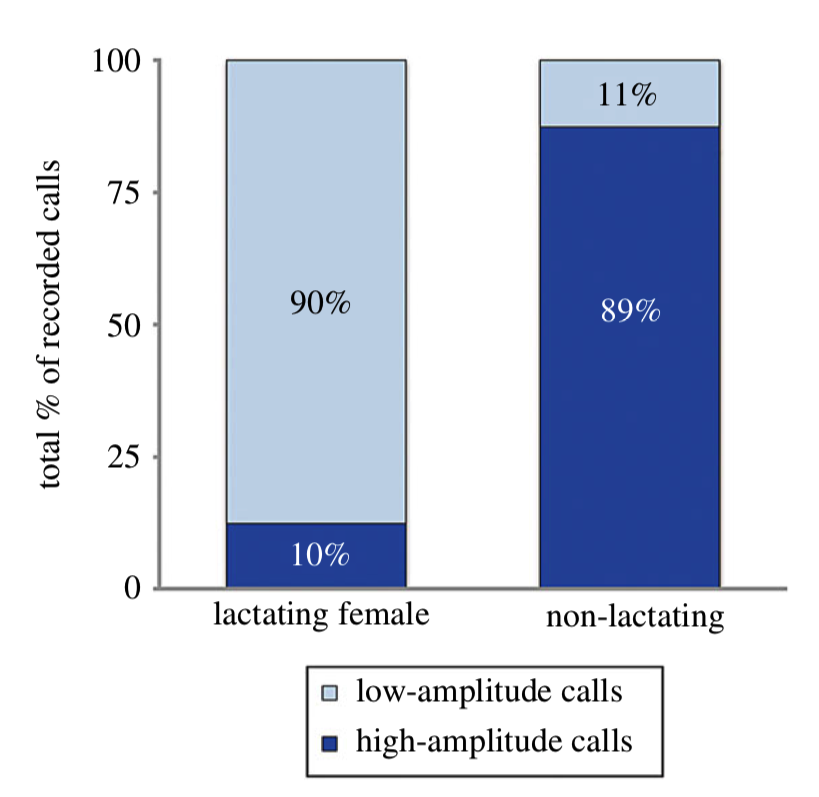
Source: Parks et al. 2019.
According to Dr. Parks, these low-amplitude sounds are analogous with human whispers (Kooser, 2019). This ‘whispering’ is a behavioral adaptation that allows communication between mother and calf without drawing the attention of undesirable predators.
Such an adaptation may seem obvious to us when we think back of our hide-and-seek game, but documentation of little details of the cryptic lives of whales is unique and fascinating. We still don’t know so much about the lives of whales, so determining adaptations, behavioral and physiological changes, and other simple features like “whispering” are crucial for us to better understand the ‘whale world’ and be able to enhance conservation efforts.
References
Guy 2017. North Atlantic right whales are going extinct. A new invention could save them. Retrieved from https://oceana.org/blog/north-atlantic-right-whales-are-going-extinct-new-invention-could-save-them. Accessed on 17 Oct 2019.
IUCN 2007. North Atlantic Right Whale. Retrieved from https://www.iucnredlist.org/species/41712/10541234. Accessed on 16 Oct 2019.
Kooser A. 2019. Right whale moms ‘whisper’ to their babies so sharks won’t hear. CNET. Retrieved from https://www.cnet.com/news/right-whale-moms-whisper-to-their-babies-for-an-important-reason/?fbclid=IwAR0JcKgYPII4a-BTjm7VPtOfjyVIb63F-SLAjyZZ2KXA6GvYJozfazcfHjA. Accessed on 16 Oct 2019.
Nielsen ML, Bejder L, Videsen SK, Christiansen F, Madsen PT. 2019. Acoustic crypsis in southern right whale mother-calf pairs: infrequent, low-output calls to avoid predation? J. Exp. Biol. 222:jeb190728.
NOAA 2019. North Atlantic Right Whale. NOAA Fisheries. Retrieved from https://www.fisheries.noaa.gov/species/north-atlantic-right-whale. Accessed on 16 Oct 2019.
Pace III RM, Corkeron PJ, Kraus SD. 2017. State-space mark-recapture estimates reveal a recent decline in abundance of North Atlantic right whales. Ecology and Evolution 7:8730–8741.
Parks SE, Cusano DA, Van Parijs SM, Nowacek DP. 2019. Acoustic crypsis in communication by North Atlantic right whale mother-calf pairs on the calving grounds. Biology Letters 15:20190485.
Taylor JKD, Mandelman JW, McLellan WA, Moore MJ, Skomal GB, Rotstein DS, Kraus SD. 2013. Shark predation on North Atlantic right whales (Eubalaena glacialis) in the southeastern United States calving ground. Marine Mammal Science 29(1): 204–212.
Thomas PO, Reeves RR, Brownell RL. 2016. Status of the world baleen whales. Marine Mammal Science 32:682–734.
Tyack PL. 2000. Functional aspects of cetacean communication. In Cetacean societies: field studies of dolphins and whales (eds J Mann, RC Connor, PL Tyack, H Whitehead), pp. 270–307. Chicago, IL:University of Chicago Press.
Videsen SKA, Bejder L, Johnson M, Madsen PT. 2017. High suckling rates and acoustic crypsis of humpback whale neonates maximise potential for mother-calf energy transfer. Funct. Ecol. 31:1561–1573.
Williams 2019. Right whale grandmother known as Punctuation killed by ship strike. Retrieved from https://www.cbc.ca/news/canada/nova-scotia/north-atlantic-right-whale-punctuation-died-after-ship-strike-1.5191987. Accessed on 17 Oct 2019.













































You must be logged in to post a comment.